Strengthening regulatory capacity in sub-Saharan Africa
Global Health EDCTP3 is supporting six projects with a total budget of €3.5 million to support sub-Saharan African countries to establish and develop robust capacities for national medicines regulatory systems.
Clinical trials and studies to investigate new or improved health technologies, interventions and medicines are fundamental to improving health and wellbeing globally. These studies must be conducted according to national and international ethical and regulatory standards. In sub-Saharan Africa (SSA), there is a need to better understand and tackle the challenges that national competent authorities are facing in overseeing the conduct of such studies. These challenges are to be addressed at different phases of clinical trial activity and medicinal products control, as well as certification and accreditation of ethics and regulatory bodies, in compliance with common international standards and open data access. Moreover, ethics and regulatory oversight in SSA countries requires national prioritisation and ownership to ensure sustained strengthening with a long-term perspective.
Building on work done under the previous EDCTP programme (EDCTP2), last year Global Health EDCTP3 launched a call under its Work Programme 2022 (HORIZON-JU-GH-EDCTP3-2022-CALL1-01-05) to support the strengthening of regulatory capacity in SSA. Six projects were selected for funding to achieve this goal:
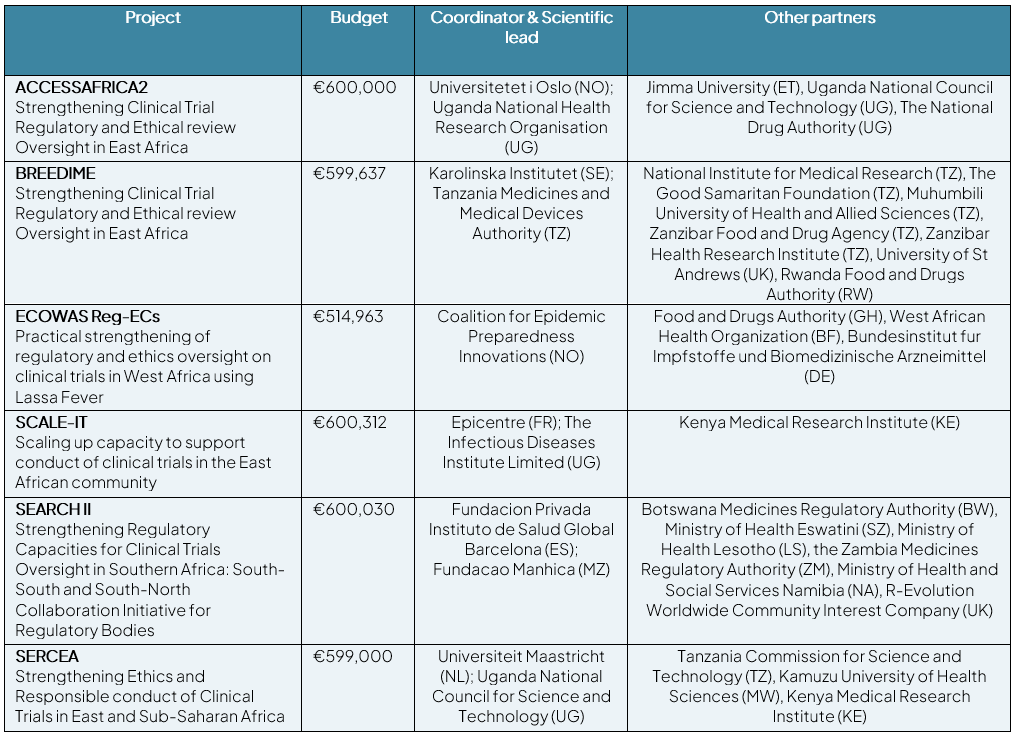
The projects will work to develop new approaches and provide training to develop personal and institutional capacities on clinical trial oversight and pharmacovigilance, targeting National Regulatory Agencies (NRAs) staff, researchers, clinicians and other healthcare workers. The training will include knowledge exchange through South-South and North-South partnerships. Moreover, the projects will promote international cooperation in regulatory activities through transfer of promising and successful innovative systems and will create linkages between regulatory functions with other important structures, such as clinical trial registries, whilst simultaneously enforcing the sharing of data in compliance with global requirements.
To continue working towards achieving the goal of a sustainable and strong regulatory and ethics system in SSA countries, a subsequent call (HORIZON-JU-GH-EDCTP3-2023-01-05) on strengthening ethics and regulatory capacity was launched in 2023 with a total budget of €8 million.
By developing ethics, regulatory and legal capacities in SSA countries, Global Health EDCTP3 will enhance the researchers’ ability to design, conduct, analyse and disseminate the results from clinical research studies, key to developing and improving health technologies and interventions to tackle infectious diseases in the region and across the world.
Background
The Global Health EDCTP3 Joint Undertaking invests in global health through funding collaborative research and innovation projects tackling infectious diseases and supporting activities for research capacity building in Africa.
Global Health EDCTP3 builds on the first and second European and Developing Countries Clinical Trials Partnership (EDCTP) programmes. The EDCTP2 programme has supported 46 projects to strengthen national ethics review and regulatory capacities in 38 sub-Saharan African countries. More information on the clinical research capacity development projects funded under EDCTP2 can be found in this dedicated publication.
Further information on the Global Health EDCTP3 annual strategy and funding opportunities can be found in the dedicated annual Work Programmes.
All calls for proposals are published on our Calls for Proposals page and on the European Commission’s Funding and Tender opportunities portal.